Early Stage and Complex Designs
Sample size & operating characteristics for Phase I, II & Seamless Designs (MAMS)

Robust Trial Outcomes
Multi-Stage Designs
Find sample size and optimal futility exit rules for Phase IIa designs using methods such as Simon’s Design, Fleming’s Design and Litwin’s Designs
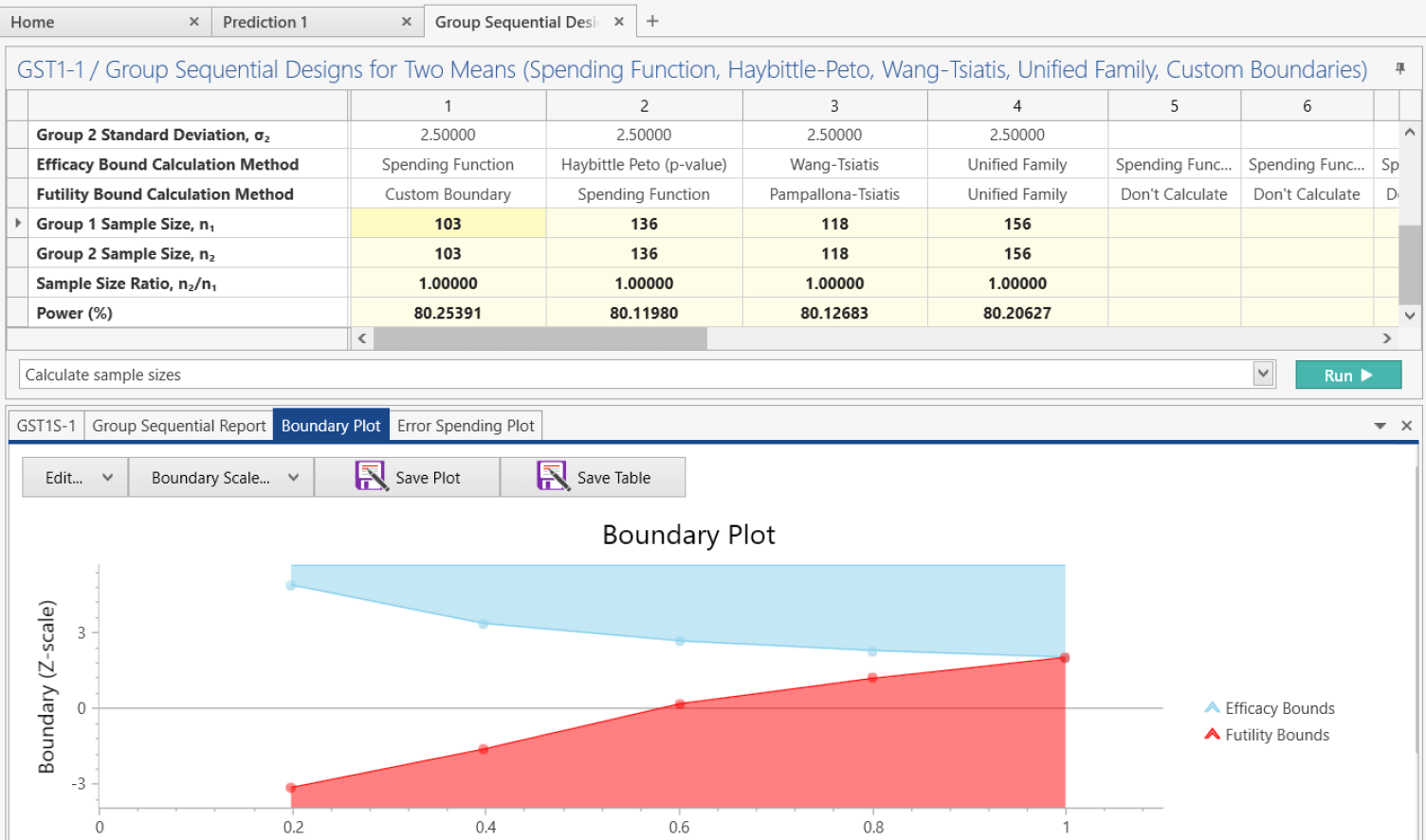
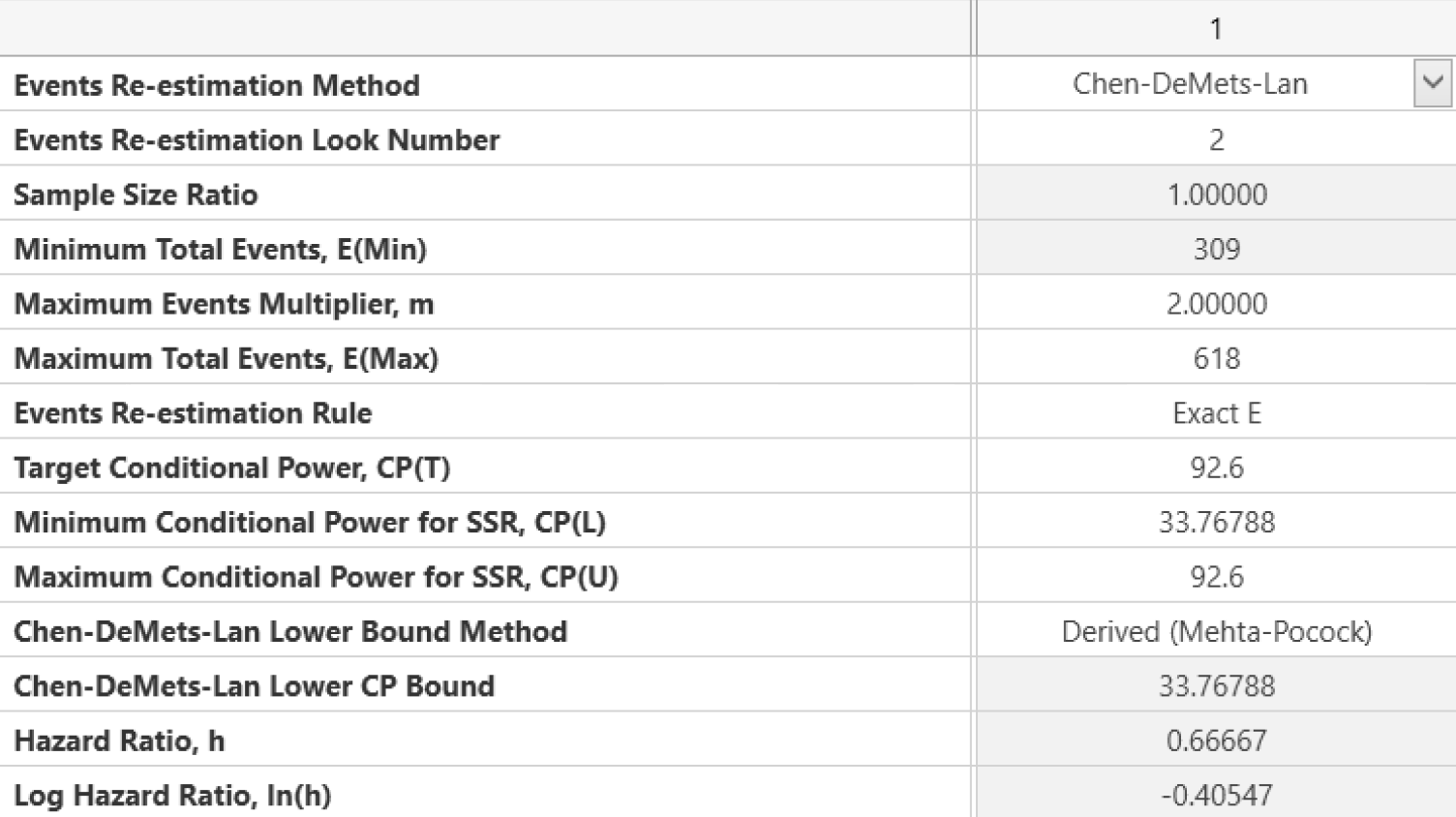
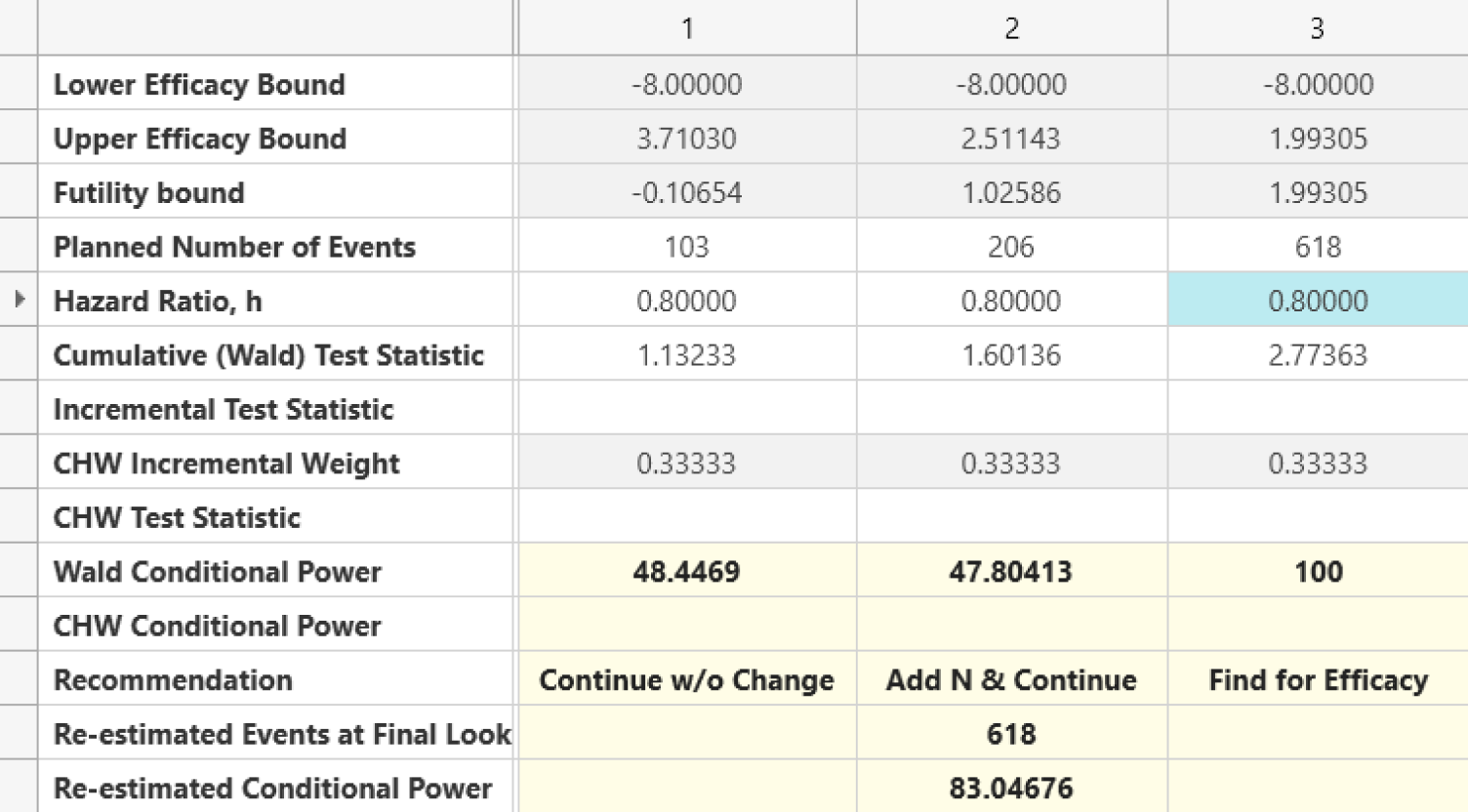
Seamless Transitions
Multi-Arm Multi-Stage (MAMS) Designs to select doses for Phase III or MCP-Mod to consolidate proof-of-concept and dose-finding stages in Phase II trials
Study Size for Phase I MTD
Find the sample size needed to find the maximum tolerated dose using the continual reassessment method (CRM)
Design Adaptive Early Stage and Seamless trials to ensure quick decisions and reduced costs
Futility Analysis at Phase IIa
Stop unpromising therapies fast using methods such as Simon’s Design
Evaluate Phase II Toxicity
Use Bryant & Day to provide stopping rules based on efficacy and toxicity
Test PFS at Phase II
Litwin’s Design extends Phase II evaluation to survival outcomes
Combine IIa/IIb with MCP-Mod
MCP-Mod merges proof-of-concept and dose-finding into single robust procedure
Seamless II/III MAMS Designs
Use MAMS methods to drop unpromising treatments or doses before Phase III
Phase I MTD
Find sample size needed for continual reassessment model (CRM)
Multi-Arm Multi-Stage Design (MAMS) in nQuery
MAMS trials can provide a quick and efficient evaluation of multiple treatments,
targets or doses under a single study protocol
Frequently Asked Questions
What is the significance of early-stage designs in clinical trials?
Early-stage designs in clinical trials focus on investigating the safety, efficacy in humans and find the optimal dosages of new treatments for evaluation in confirmatory Phase III clinical trials. The design choices in early-stage trials can play a crucial role in determining the viability of further development.
Phase I trials focus on establishing the safety profile of a treatment, typically by finding the maximum tolerated dose (MTD) using methods such as i3+3, continual reassessment method or mTPI-2.
Phase II trials seek to find an efficacy signal and select the appropriate doses for Phase III. Phase II is often split into Phase IIa (proof-of-concept) and Phase IIb (dose-finding) trials.
What types of early-stage designs can be accommodated by nQuery?
nQuery supports a range of early-stage designs.
For Phase I, nQuery supports one-arm trials alongside flexible models such as CRM.
For Phase II, nQuery includes tools for fixed term trials plus common multi-stage Phase IIa designs such as Simon’s, Bryant & Day and Litwin’s.
nQuery also includes MCP-Mod which combines proof-of-concept and dose-finding into a single procedure.
Recommended Resources
Get started with nQuery today
Start for free and upgrade as your team grows